© 2007 All Rights Reserved. Do not distribute or repurpose this work without written permission from the copyright holder(s).
Printed from https://www.damninteresting.com/coleys-cancer-killing-concoction/
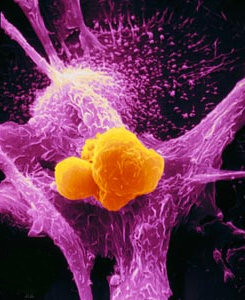
On October 1st 1890, William B. Coley, a young bone surgeon barely two years out of medical school, saw one of his first patients in private practice at the New York Memorial Hospital. Although he’d only finished his residency earlier the same year, he’d already gained a good reputation and many considered him a rising star of the New York surgical scene.
The seventeen year old patient had a painful, rapidly growing lump on the back of her right hand. She had pinched the unlucky appendage between two railway carriage seats on a transcontinental trip to Alaska some months before, and when the bruise failed to heal she assumed the injury had become infected. However the bruise turned into a bulge, the pain steadily worsened, and her baffled doctors were eventually compelled to call for Dr. Coley. As a surgical man, Coley would never have guessed that this innocuous referral would take his career in a totally new direction— into an unusual branch of medicine now known as cancer immunotherapy.
At first Dr. Coley was also uncertain about the diagnosis. But as the girl’s condition rapidly deteriorated– with the lump becoming larger, more painful, and associated with the loss of sensation in some of the surrounding skin– the awful truth became apparent. She had a sarcoma, a type of cancer that affects bone and connective tissue in the body. Unfortunately, 19th century medicine offered very few treatment options.
On November 8th, Coley amputated her arm at the elbow. Although the operation appeared to go well, the girl– named Elizabeth Dashiell– developed severe abdominal pain three weeks later. Soon thereafter she noticed more lumps in her breasts and armpits, signs that the cancer was metastasizing, or spreading. She rapidly lost strength and died on January 23rd 1891, a scant three and a half months after her initial consultation, with a traumatized Dr. Coley at her bedside.
Elizabeth’s death hit the young surgeon hard. While a more experienced physician might have shrugged away the apparent failure and moved on, Coley was determined to do something. His ensuing efforts culminated in the development of a famous fluid that, for a time, appeared to promise the fulfillment of that long-held dream: a universal cure for cancer.
Coley began by poring through the hospital’s records, looking for clues from previous sarcoma cases that might lead to better treatments in the future. He soon found what he was looking for: the case of a German man who came to the hospital with an egg-sized sarcoma in his left cheek some seven years earlier. There were several attempts to excise the tumour but none of them were successful— each time the cancer came back, as aggressive as before. The final operation could only partially remove the huge mass, leaving an open wound that subsequently became infected. The unfortunate immigrant was deemed a terminal case.

Yet four and a half months later, the man was discharged with no trace of disease. Coley personally tracked down the former patient to verify that the miraculous cure had taken place. Indeed, the man was healthy and happily settled into his new life in the United States. The records showed that after the wound became infected with a commonplace bacterium, Streptococcus pyogenes, the patient went through several bouts of fever. With each attack of fever the tumour shrank until eventually it disappeared entirely, leaving only a large scar under the left ear. Coley surmised that the infection had stimulated the German’s immune system– as evidenced by the repeated fevers– and that it was this immune response that had caused the eradication of the cancer.
The story so convinced Coley that he– perhaps cavalierly– contrived to contaminate his next ten suitable sarcoma cases with Streptococcus. His initial approach was to inject a solution of live bacteria deep into the tumour mass on a repeated basis over several months. The first patient to undergo this treatment was a bedridden man with inoperable sarcoma in the abdominal wall, bladder, and pelvis. Using this experimental method, the patient was cured spectacularly. He staged a full recovery, and survived another twenty-six years before dying from a heart attack. But subsequent results were mixed; sometimes it was difficult to get the infection to take hold, and in two cases the cancer responded well to treatment but the patients died from the Streptococcus infection.
Coley’s discovery, as it turns out, was actually a re-discovery. The idea of a link between acute infection and the resolution of tumours was not new, and the phenomenon of infection-related “spontaneous regression” of cancer has been documented throughout history. A 13th century Italian saint was reputed to have his tumour-afflicted leg miraculously healed shortly after the malignant growth burst through the skin and became infected. Crude cancer immunotherapies working along similar lines to Coley’s early experiments were known in the 18th and 19th centuries, and may extend back to the time of the pharaohs. Ancient writings suggest that the renowned Egyptian physician Imhotep may have used a similar infect-and-incise method to treat tumours.
But Coley took those first important steps in dragging this old remedy into the twentieth century. After the fatalities with the ‘live’ version of his therapy, he developed an improved fluid containing killed bacteria of two different strains, Streptococcus pyogenes and Serratia marcescens. This was based on the idea that the dead bacteria would still have the immune-stimulating capability of their living brethren (in the form of purported ‘toxins’), but not share their inconvenient tendency to cause death.

His invention became variously known as ‘Coley’s Toxins’, ‘Coley’s Vaccine’, ‘Mixed Bacterial Toxins’ or ‘Coley Fluid.’ The treatment was met with considerable success, with one study in 1999 suggesting that it was at least equally as effective in treating cancer as conventional modern therapies. With due care in dosing and management of the induced fever, it was also remarkably safe.
Although Coley took the concept of immunotherapy much further than his pharaonic forebears, he had no clear idea how his toxins actually worked, and the tools did not yet exist for him to find out. But given the rapid scientific progress at the turn of the last century, he reasoned that a deeper understanding of his therapy would arrive soon enough. Although the true extent of his “Toxin” success has been questioned by historians, the validity of his approach has never been seriously called into doubt. Indeed his results are regularly cited in the cancer research literature to this day.
Over the following years Coley continued to refine his technique. He determined that the toxins should be administered to patients at progressively higher doses to counter the body’s innate “immune tolerance” to the treatment. Other physicians in America and Europe also experimented with the method, and found that the toxins appeared to work just as well in a number of different non-sarcoma cancer types such as carcinoma, lymphoma, and melanoma. They could also be given intravenously some distance from the site of the tumour, and still be effective. Variations on the basic bacterial recipe and different dosing regimes were tried, depending on the individual patient and the particular cancer’s type and proliferation. Through his career Coley himself treated over one hundred patients with his concoction, and countless more were treated by other doctors.
As the fame of his fluid grew, so did Dr Coley’s stature: in 1915 he became head of the Bone Service at the New York Memorial Hospital (which later became the Memorial Sloan-Kettering Cancer Center). By the time he died in 1936, Coley’s Toxins were mentioned in a number of different surgical textbooks as a standard anti-cancer therapy.
Conventional modern medicine, however, very rarely employs Coley’s Toxins in the treatment of cancer, for reasons almost as complicated as the human immune system itself. One concern is the far-from-complete understanding of the mechanism of action; generally, doctors are reluctant to administer treatments whose workings they don’t fully comprehend. The stimulated human immune system is a whirling tempest of different physiological and biochemical responses, and even now there’s much uncertainty about how Coley’s Toxins modified this complex mechanism to better attack its cancerous target.

One theory stresses the importance of the fluid-induced fever in killing the cancer cells; another considers the debris-engulfing macrophage cells to be the main players, while others consider various different immune messenger molecules— or cytokines— to be important.
The eclipsing of Coley’s Toxins also had something to do with the concurrent development of radiation therapy and, a little later, chemotherapy. Soon after Wilhelm Roentgen discovered X-rays in 1895, the possibility of using radioactivity to treat cancer was investigated. The technology was exciting, new, and developing fast along well-understood principles. Although the first results of radiotherapy weren’t all that impressive, it had the advantage of fractional doses, and once the equipment was in place it didn’t require the complicated, patient-specific preparation which was needed with Coley’s Toxins. Likewise chemotherapy was based on known scientific principles, and could be manufactured and used relatively easily.
Furthermore, both radiotherapy and chemotherapy have an immune-suppressing side-effect. Since both treatments kill the rapidly dividing cells of the immune system along with the rapidly dividing cancer cells, both can be used together if care is taken. But immune-stimulating Coley’s Toxins work entirely differently, and their effect would be cancelled out if used at the same time as high-dose immunosuppressant chemo- or radiotherapy. It became an either/or situation— and in the end, the fashionable new treatments won out over Coley’s fiddly reworking of an ancient ‘natural’ remedy.
So when the US Food and Drug Administration changed the status of Coley’s Toxins to that of a ‘new drug’ in 1963– meaning that it could only be used in clinical trials, and greatly reducing its availability– it seemed that its time had already long passed. But cancer immunotherapy does have limited applications today. Perhaps its most frequent mainstream use is in the treatment of bladder cancer; solutions containing the tuberculosis vaccine are routinely instilled into cancer-affected bladders, and are effective in causing regression of tumour deposits. It is theorized that the bladder’s immune response deals with the cancer in a similar way to the whole-body immune effect of Coley’s Toxins.

Melanoma, a particularly nasty type of skin cancer that responds poorly to conventional radiotherapy and chemotherapy, is sometimes treated with an immune-stimulating cytokine called interferon.
In some ways this century-old form of treatment is still a fringe area of medicine. But researchers have once again begun to probe the possibilities of immunotherapy. New antibody-based treatments like Mabthera and Herceptin are making a real difference in the treatment of common cancers like lymphoma and breast cancer. Although these therapies don’t stimulate the body’s immunity as a whole, they are based on antibody molecules which are key components of the human immune system. They show that our increasing knowledge of the molecular nitty-gritty of the body’s own defence and repair network is starting to make a real difference in the battle against cancer. One tumour at a time, such advances in modern medicine are finally vindicating William Coley and his one-hundred-year-old cancer-killing concoction.
© 2007 All Rights Reserved. Do not distribute or repurpose this work without written permission from the copyright holder(s).
Printed from https://www.damninteresting.com/coleys-cancer-killing-concoction/
Since you enjoyed our work enough to print it out, and read it clear to the end, would you consider donating a few dollars at https://www.damninteresting.com/donate ?
Sometimes the more things change the more they stay the same. Bad grammar but you get the point.
Another interesting story, thanks!
so close
Damn Interesting! I wonder why I’ve never heard of this – I’m not a doctor, but I’m no slouch in my understanding of health issues and common treatments.
If I had cancer, I think I’d rather have Coley’s cure than radiation and chemo.
The most recent issue of Scientific American has some interesting related stuff on how cancer hijacks the immune system to help itself grow and metastasize at the same time that other parts of the immune system are attacking the cancer. Very interesting stuff.
Another DI blog…thanks
wh44 i was thinking the same thing, i would also prefer this to chemo
I’d prefer whichever kept me alive.
I wonder though, how much did politics and perception have to do with the decline of coley’s treatment and the subsequent rise of radiotherapy?
Damn Interesting, thank you. We’re getting closer and closer to beating cancer, I’m sure we’ll get there soon.
Agree with wh44 and deco05ie, I think in many ways Chemo can be worse than the cancer itself and I would much rather go through a few serious fevers that one can recover from properly. Each individual case is different though, but it seems that it would be appropriate for this kind of therapy to be more widely acknowledged so that patients have a better choice.
I worry that cost-cutting exercises by health services might lead to a ‘one-size-fits-all’ approach, with more complex treatments (that also undermine the physician’s knowledge and prestige as they don’t understand properly how it works) such as this one at the worst actively discouraged, and at the least disfavoured.
With government health services and doctors treating so many patients on a daily basis and being so familiar with death they have no choice but to value a life less than an inexperienced person would. Whilst this is clearly appropriate from an objective perspective to help save more lives than are lost, it is clearly not an approach that would be considered had we unlimited resources to spend.
One day hopefully the billions poured into defence and the creation of nuclear deterrents and so forth will be redirected into health and welfare services. One day….
Joe BB
The ability of the human body to repair and restore is amazing, even when it takes a slight nudge from something else to get it in gear.
Having been exposed to two different types of radiation, and going through the post-treatment/analysis, I can tell you the aftereffects suck royal. Now I live day-to-day knowing that I can at anytime suffer some type of cancer. My choice for treatment would be Coley’s cure. Though Chemo helps retard and even stop a cancer, it also damages healthy cells during the treatment.
When people hear the word “Radiation” they usually think of radioactive isotopes, like those in a nuclear reaction. My radiation exposures were from a more common source. The first was when over 15,000 volts burned a hole into my thumbnail, traveled through the core of the bone, and exited out my elbow. The second was exposure to high levels of RF radiation when during a safety survey of a transmission tower, the control personnel misunderstood my command of 8% power and zapped me with 88% power. This created an RF bubble that “cooked” my arm. It turned bright red as if I had sunburned just it. All the hair fell out (it looked really strange, like I had a discolored prosthetic).
In both cases, my DNA may have been damaged in any of the cells that were in these locations at the time. As I age, this could be a lethal time bomb ticking away in any of thousands of cells in my body as the DNA helix decays. Do I worry about it, naw. We all finally die. Just in different ways. So everyday I go through my body examination routine. Looking for the signs of DNA failure. Then it’s off to enjoy another exciting day of adventure. Why live in the dark when there is so much sunshine you can enjoy.
After all, is not the celebration of a birthday not only the achievement of one getting older, but also the celebration of one step closer to the grave?
wh44 said: “Damn Interesting! I wonder why I’ve never heard of this – I’m not a doctor, but I’m no slouch in my understanding of health issues and common treatments.”
In my minor in biology I never heard this, and I took a number of genetic and microbial courses. I can’t even fathom the mechanism of this but what I think could be happening is that the caner cells don’t have the proper antigens on their membrane. They probably don’t have any at all considering how a cancerous cell develops. Usually they fly under the radar, they aren’t considered dangerous because they don’t have anything that the immune system considers bad on their membrane. Then comes the infection. The body is suddenly put under marshal law, while taking out the bacteria, which have a foreign and hostile antigens on their membrane, the macrophages start to consume the cancerous cells as well. The cancerous being unknown and possibly dangerous. Kill them all and let the body sort them out.
It is an interesting hypothesis, probably complete bullshit.
I would be so curious to figure out the mechanism in this immunotherapy. I would rather be sick and delirious in my bed fighting off a strep infection then being irradiated.
Always,
Craven Morhead
Cancer is a well-known subject in my family and having experienced it myself along with the radiation and chemotherapy I can attest to the extremely harsh effects of them both. To give an example, while it was being administered to me and being an infant, I moved and it spilled on to my skin giving me a third-degree burn.
I’ve also assumed that the radiation passed over my spine stunted my growth- I had cancer on one side of my abdomen and rather than having the radiation hit just part of my spine the doctor opted to run the radiation across my entire abdomen to cover it evenly as to not stunt one side of my spine. Not being a doctor or an expert on radiation I don’t know how much truth that holds as this was 30 years ago, too.
My only indication that there was SOME truth in this is the fact that I have constant back pain in that area when I lean over slightly and also the fact that my brothers are a lot taller than I am. Again, could be happenstance.
Before treatment of chemotherapy they must take your vitals to see if your fit for each treatment, that to me is a good indication of how damaging it can be.
Dear Matt..and Alan…you realize through your efforts that you could save lives? I think what you do with DI is excellent. I’m going to go Digg this to make sure more people know about it….DI Friends..please help.
I imagine that in 100 years, civilization will look back at us and say, “They used radiation?! To cure cancer?! What were they thinking???”
Thank you, Matt. . . spectactular and DI article. I have never heard of this and think it’s a great idea. It is moronic that we now wipe out the body’s ability to defend itself and intervene with our powerful synthetic drugs. It makes much more sense to stimulate our body’s defenses. The body is a wonderful mechanism and we should treat it like an ally in the battle against cancer rather than an incapable child who we butt aside and say ‘HERE, let ME do it’
I just noticed that I posted the thirteenth comment on Friday the 13th. I’m DOOMED.
This article finally prodded me into registering, just so I could get the opportunity to comment.
Good news, joefyn and Radiatidon. There are working “Coley’s Toxins” in use today!
I was diagnosed with malignant melanoma in February of 2003. In my initial meetings with my oncologist, he introduced me to a “new” drug that had the highest known success rate at the time. This drug was developed from the interferon studies, and uses a naturally-occuring chemical in the body called interlukin. The drug is Interlukin II – a lab-manufactured interkukin “super booster”. Instead of tearing down the immune system, like chemotherapy, it super-charges it. One of its effects is to make the white blood cells even more aggressive in killing the cancer cells.
Prior to starting the treatment, I was told about the relatively poor results of traditional chemotherapy and radiation treatment. And having watched my own mother and grandmother pass away after their own experiences with chemotherapy, I expected the worst.
I am happy to tell you two things: One, it wasn’t that bad. Each treatment cycle was like having the flu for a week. Two, it worked spectacularly. I have been cancer free since November of 2005! Do I consider myself lucky? You’d better believe it! The mortality rate for patients who are treated for melanoma is less than 10%! If I remain cancer free for another three years, there is very little chance that it will ever return!
Interlukin II isn’t for every type of cancer, but as I can attest myself, it works very well on melanoma. There are other “Coley’s Toxins” being developed today that focus on other types of cancer. I know that there is another drug available for pancreatic cancer, for breast cancer, and for several other soft-tissue cancers.
Thank you Matt…and Alan. And I hope that any DI readers who first hear their doctor utter the dreaded “C” word get a chance to read this article. There really is hope out there.
p.s.
Has anyone submitted an article about gamma knife procedures? If not, I’d be interested in working on such an article. It reminded me of something that Dr. Leonard McCoy might have administered…
This is a excellent article to see for me. I have been looking into more natural style medicines, therapies, foods and such. Thus far I have seen similar stories and articles where simpler more effective ways are shoved aside because (it would appear) there is no money in it. Not like in chemo/radiation, etc. type ways. Success seems to take a backseat to profitability. Damn!
SWehrly said: “This article finally prodded me into registering, just so I could get the opportunity to comment.
…”
Thank you for your comment, and welcome to DI! It’s nice to hear an article backed up by someone’s real experience.
I know that there are a lot of people that haven’t heard of this treatment but I recently took a microbiology class that touched on the idea that certain types of infections could help kill cancer but we just barely skimmed the surface is to why it worked. Also there was an episode of House that dealt with a similar idea. Perhaps with more research this will become a more viable option
Damn Interesting Indeed!
Knowing the hell that cancer patients endure during chemo and radiation, I would opt for Coley’s cure any day.
It is nice to know other options exist.
DI, I would like to see this taken further, allthough radioligy and chemo do have their advantages as well as dis advantages. This article does seem to only look on the ‘good’ side of using pathogens to kick start the immune systems. I would like to see the negative sides of using pathogens.
I had a friend relate his experience battling cancer with a combination of chemo and low levels of arsenic common to many fruit seeds. I don’t know if this is true but my friend swears by it and continues to this day, 5 years after winning the battle. [/p]
Has anyone heard of such a thing or is this another entry in the long line of internet perpetuated misinformations?
MarshyMarsh said: ” I would like to see the negative sides of using pathogens.”
I’m not sure about other drugs of this kind, but with Interlukin II, there is a serious tax on the liver and the kidneys. While going through the treatment, I had to remain in the ICU for observation, and have blood drawn every 4 hours to monitor my bilirubin (liver) and kidney functions.
So during the treatment, I was also given other drugs specifically to keep my fluids balanced, my blood pressure stabilized, and even keep my skin from peeling off. I was monitored routinely by an endocrinologist, a heart specialist, a respiratory specialist, as well as my oncologist. As soon as either the liver or the kidney “counts” reached a danger threshold, the drug cycle was terminated for that week. Once the treatment cycle ended, I was kept in the ICU for an additional 24 hours until my liver and kidney “counts” returned to normal.
It made me speculate that these counter-measures in the ICU were adopted because previous patients had almost died on the drug.
These “negative” conditions were only a concern while I was being administered the drug. As an out-patient, my blood tests always showed normal levels, and no imminent dangers.
The only long term side effects I have experienced since treatment is heightened allergies to air-borne particulates (smoke, smog, perfume, etc.) and greater sensitivity to the sun (but since I had melanoma, I must avoid being in the sun for long periods of time anyway). It’s been almost two years now, and no other side effects have surfaced. I realize that 2 years is too short to call it “safe”. I’m looking forward to year 5!
One positive side effect is that with a super-charged immune system, I get over colds much quicker than before!
“If I remain cancer free for another three years, there is very little chance that it will ever return!”
My thoughts and prayers are with you for a cancer-free life.
wh44 said: “Damn Interesting! I wonder why I’ve never heard of this – I’m not a doctor, but I’m no slouch in my understanding of health issues and common treatments.
If I had cancer, I think I’d rather have Coley’s cure than radiation and chemo.
The most recent issue of Scientific American has some interesting related stuff on how cancer hijacks the immune system to help itself grow and metastasize at the same time that other parts of the immune system are attacking the cancer. Very interesting stuff.”
Yes… I really can’t comprehend why doctors actually want to supress the immune system… it makes way more sense to stimulate it…
Quoting Star Trek, “Sounds like the damn Spanish Inquisition.”
Great article DI! Has anyone read about that Dr. that cured her own cancer? I haven’t verified or explored any of her claims, but if I were sick, I would check everything out! (Even green eggs and ham!)
Just one quick little note…I am a newbee to DI and have been extremely impressed by the awesome articles and the very interesting comments all of you have been posting. I normally do not bother reading blogs because they can be extremely irritating, but this one is different. I LOVE TO READ everyone’s comments. (I love the various points of views!) The comments are either educational, uplifting and sometimes pretty darn funny. Please keep it up! I LOVE YA’ll, MAN! (warm fuzzies)
Great article,Matt!
I agree whole heartedly with Nicki and Craven Morhead..;
(LOL, I just now after all these months, caught the pun in your moniker, too funny.)
“The most natural way to heal is to allow the body to naturaly heal.” How simply profound is that?!
Thank you too, to all the new commentors who have shared their stories and views. This is what makes this Damned Interesting site the best in the www!
Like Miss Davida says, lets spread the word,
and while your at it, check out the upper right hand corner of this page & click the link….
There’s gonna be a book! Yeah, Alans writing a book!
It will make a great gift! Contribute today,to reserve your copy fresh off the press!
;-)xxxooo
Excellent article! Another way *anyone* can help fight cancer and other deadly diseases with their own PC is by downloading BOINC and attaching to these projects:
http://boinc.bakerlab.org/rosetta/
http://predictor.scripps.edu/
http://biology.polytechnique.fr/proteinsathome
http://boinc.bio.wzw.tum.de/boincsimap/
http://issofty17.is.noda.tus.ac.jp/index_E.php
http://www.worldcommunitygrid.org/
http://www.malariacontrol.net/
I just read an article (somewhere, not sure where) that stated that people with some dormant varitions of Herpes are at a diminished risk from cancer. I think it was a SciAm, maybe Discover from last month– one of those little snippets they put in. I didn’t bother checking up on it–though I suppose now I must.
To everybody who said that they would rather be infected than submit themselves to radiation treatments (I am not a doctor so I could just be misinformed): The bug C sucks no matter what. I would have to assume that medical professionals have previously weighed the risks and benefits of each different treatment ( Coley’s Toxins WERE used in the past, and documented, mind you) and made an educated decision on which were most effective.
Having said that, it is never too late to step back, re-examine the problem and possible solutions, and choose a different tact. I’m just asking you not to assume that this treatment is not the end of cancer, merely (PERHAPS) a means to the end of it.
Wow! So many people are going to know about this option to Chemo and Radiation. DI… and very helpful.
tednugentkicksass said: ” I’m just asking you not to assume that this treatment is not the end of cancer, merely (PERHAPS) a means to the end of it.”
Sorry, that made no sense. I meant: “I’m just asking you not to assume that this treatment is the end of cancer, merely (PERHAPS) a means to the end of it.”
How about fighting cancer with an ancient form of chinese tea?
Extract from article –
“Green and black Yunnan Pu-erh teas were found to have the ability to destroy cancer cells – Yunnan Natural Medicine Research Institute”
For those interested this is the article I found >>
http://www.puerhcha.com/Health/Puerh_Tea_Health2.htm
I don’t really understand why a safer treatment with less severe side-effects was replaced by potentially dangerous treatments that mess with the immune system. I hope more research is done in immunotherapy, because god damn it, it sounds almost like a miracle cure.
Sulevis, stimulating the immune system can be as dangerous as suppressing it.
Keep in mind, things like allergies are just your super-charged immune system going crazy.
Ted I have read some of the same articles about viruses proving to be an effective treatment on cancer. Herpes being one that seems to work well on both lung and brain cancer. As a matter of fact, if you click on the related article link “Cancer Assassins” at the end of this article, you will see just that.
I have a gentleman that works for me that was diagnosed with Stage 4 intestinal and liver cancer back in February of 2005. His cancer counts were right about 4,000 when first diagnosed. As of the end of last year, after tons of chemo and radiation, they had gotten it down into the 300’s range (normal they say is about 10). He had heard about some therapeutic mushroom pill made in Hawaii and started taking them. After checking and rechecking, this May he was cancer free…he thinks it was the pills.
Sadly he stopped taking them and earlier this month they have found a small amount of cancer now on his aorta. I’m pretty worried about that, he’s a great guy and has just kept the best attitude throughout his entire ordeal. For the last two years anytime I call him and ask how he is I get the same standard response “Alive and kickin, that’s half the battle”. I’d like to think if it were me I’d carry myself the same way.
I’d really like to think they can keep working on research like immunotherapy and find easier, more exact ways to kill the stuff and sooner rather than later.
I just had to post. This really bothers me. I visited my grandma yesterday, who has ovarian cancer and is receiving chemotherapy. She is slowly wasting away, and it was horrible to see. I left there crying and thinking there has to be a better way than killing all of these good cells in her body and destroying her immune system. I am a huge believer in building up your immune system, and the idea that the body can heal itself. I have recently become very leery of western medicine and their ways of treating patients, covering up symptoms with drugs instead of treating the whole body and the whole person. Especially watching my grandma these last 6 months. I believe that there are better ways, but politics and money have altered the way that traditional doctors deal with many illnesses, not just cancer.
If I found out I had cancer, I would seriously look into every possible alternative before going with chemo or radiation.
Thanks for a DI article.
Dylanfan, I am sorry to hear about your grandmother. I saw my own grandmother and mother “waste away” in the same manner.
It really does seem unnecessary, but before you give up hope that there are doctors out there who care, check out these two sites:
http://www.jwci.org/ John Wayne Cancer Institute
http://www.CityOfHope.org City of Hope
When I was diagnosed, I traveled to both City of Hope and JWCI and met with two of the top oncologists in the U.S. for consultation. At JWCI, they are involved in trials with drugs that go even beyond what I was given.
These two institutions have been around for decades, and they are front and center in the battle against all forms of cancer. City of Hope includes doctors from around the world, so western medicine solutions are not the only tools they use.
In your grandmother’s case, I can only speculate that her oncologist was strictly “by the book”, and not interested in alternative solutions, or he offered her the best solution given her age and state of health. I know that if I had been thirty years older with weakened kidneys, or a smoker, or an alcoholic, I’d be pushing up daisies right now. The drug I was given, Interlukin II, is only given to people who have healthy livers, hearts, and kidneys.
For anyone else who may face a journey with cancer, or have a loved one diagnosed with it, there is new hope every day. New “miracle” drugs are being developed every month at places like JWCI and City Of Hope (and dozens of other research centers world wide). Doctors have gotten smarter, they are no longer looking for that “silver bullet” that will cure all cancers, they are focusing on each type of cancer as a separate disease.
Technology is improving their odds. The PET scan is one of an oncologist’s best tools. It looks for “hot spots” in the body. Cancer cells have an elevated metabolic rate, and light up like Christmas tree lights on a PET scan. Doctors are able to find cancers in their earliest stages, even before they metastacize.
(But please, don’t go and order your doctor to give you a PET scan just because you’re anxious about cancer. Let the doctor do the other preliminary blood work first. Too many people are getting PET scans without the diagnosis first, and insurance companies are starting to reject orders for them. This robs true cancer patients, and their doctors, of the best tool they can use to fight the disease!)
If you are over 40, or have a family history of cancer, or (like poor Radiatidon above) experienced something in your life that could cause cancer, get diagnosed by an oncologist. Early detection is absolutely your best defense!
I was fortunate in having an oncologist who was raised and educated in Pakistan and India, and had served time in leading cancer institutes throughout the U.S. He stayed informed of all of the trials, and consulted on my case with other leading oncologists. Not only am I confident that I received the best care I could (on an HMO), but my current health condition confirms it.
Alternatives are out there, DI fans.
Bless you all. Here is an interesting news bit from yesterday, my Badder-Mienhoff moment of the week:
New breath test detects lung cancer
http://news.yahoo.com/s/nm/20070713/sc_nm/lung_cancer_dc_2
NEW YORK (Reuters Health) – Testing exhaled breath with a small sensor array can detect lung cancer,,,
Hey Matt, great article!
I’m now 41 and you know you’re at a milestone when you’ve had multiple friends with the big ‘C’. A friend of mine here was treated for cancer of the bladder, and now he goes through multiple sessions where Tuberculosis ‘juice’ (I’m sure it’s some type of semi-dormant TB) is injected down the urinary tract of his shmeckie and then he has to hold it in for quite some time. It gives him a low-grade fever and such, but they swear by this treatment.
I agree that the immune system should protect you from all sorts of nasties– it just makes sense to boost it to cure people instead of poisioning with Chemo or baking them with Radiation.
I just keep thinking about how kids are staying indoors so much nowadays and not playing outside as much. It seems to me that running around in the woods, catching frogs, and swimming in creeks would initially build up the immune system to later combat health issues in the future. I don’t know if this is how it all works, but it makes sense to me. And all of the parents that teach their children to not get dirty and wash their hands 20 times a day, just seems to me that they are preventing the immune system to develop into a healthy and strong combatant. Like I said, I may not be right, but thats the way I see it. Let nature do its job.
So, from what I understand, Coley’ s Cure does work effectively against cancer, but doctors don’t use it because a) They don’t understand how it works
and b) radiatian is much flashier – and newer and therefore seems better than what went before.
My entire family died of cancer, and has done for generations (apart from the one killed by Spanish Flu, and the ones killed in war) so chances are high I’ll get it too. Having seen what chemo does (reducing strong, fit men to bags of bones barely able to move), I’d rather take Coley’s cure – but chances are I won’t be offered it (NHS tends to stick to tried and tested methods). It all seems a bit unfair that there’s a possibly better cure for cancer out there, but doctors won’t use it.
Cancer is abhorrent. Sometimes I wonder if there are better ways to battle cancer, even to cure it, out there. How much money does the health care industry make from of cancer? A better question, how would they lose if they had a simple, cost effective cure for cancer. You wouldn’t need the business of expensive chemo or radiation or MRI’s or surgeries or etc that cancer brings. I couldn’t imagine that it would be possible for something as reprehensible as that to take place, but I can’t imagine half of the injustices of the world.
llnatural22 said: “How about fighting cancer with an ancient form of chinese tea?
Extract from article –
“Green and black Yunnan Pu-erh teas were found to have the ability to destroy cancer cells – Yunnan Natural Medicine Research Institute””
And how good is that research? Has it been peer reviewed and replicated? I remember hearing about studies that said that vitamin C killed cancer cells, but when you actually read them it turned out that they were administering vitamin C directly to cancer cells in a petri dish, and not studying how it would work in the human body. More realistic research showed no effect. Personally, I wouldn’t trust a site that’s trying to sell the stuff to give a decent review of the scientific literature.
Sulevis said: “I don’t really understand why a safer treatment with less severe side-effects was replaced by potentially dangerous treatments that mess with the immune system. I hope more research is done in immunotherapy, because god damn it, it sounds almost like a miracle cure.”
The problem is that it is not clear that it is safer or has less severe side-effects, and it is definitely not a “miracle cure.” The possible side-effects include high-fever (including related risks like delirium and brain damage if the fever is not controlled,) chills, nausea, possible tissue necrosis (which would then have to be drained,) increased or decreased heart rate, and since it could possibly introduce live bacteria, infection and death. Also, since the immune system is being thrown into overdrive there may be the possibility of inducing auto-immune diseases, such as MS (admittedly this is speculation on my part.) Furthermore, treatments take place after every two days for weeks or months in increasing doses, and it requires frequent to constant monitoring of the patient. The treatment may not be as “pleasant” as you imagined. Please keep in mind that you’re also hearing more about the successes than the failures here, so it may give you a biased perspective. Also, keep in mind that there has been little research in this area in the past 50 years, so it’s hard to say how safe it could be or how it compares to other treatments, so you cannot conclude that it is safer or has less side-effects.
Cancer is a complicated disease that can come from a multitude of reasons and manifest in many forms. Anyone claiming to have a “magic bullet” or “miracle cure” for all cancer is a fraud of either the self-deluded or con-man variety. There is no one best way to cure all cancers, so it is a bad idea to say you prefer one method over another when you have no idea which works best for the particular kind of cancer you’re talking about. The idea of simple solutions may be pleasing, but it is usually not realistic in complicated problems like cancer.
EVERYTHINGZEN said: “I have a gentleman that works for me that was diagnosed with Stage 4 intestinal and liver cancer back in February of 2005. His cancer counts were right about 4,000 when first diagnosed. As of the end of last year, after tons of chemo and radiation, they had gotten it down into the 300’s range (normal they say is about 10). He had heard about some therapeutic mushroom pill made in Hawaii and started taking them. After checking and rechecking, this May he was cancer free…he thinks it was the pills.
Sadly he stopped taking them and earlier this month they have found a small amount of cancer now on his aorta.”
This is an example of both anecdotal evidence and post hoc ergo propter hoc fallacies. One should not take a look at a single example of something and then extrapolate that it is a general truth. One person may find that that they rubbed lemon on a burn and it healed, but that doesn’t mean that this would work for most other people, or even anyone else. The other problem there is that rubbing the lemon may have nothing to do with the healing process. The “post hoc ergo propter hoc” fallacy means that just because B came after A does not mean that A caused B. Some people may hear things like, “He was sick, I prayed, and he got better,” and assume that the prayer helped. However, substitute “brushed my teeth” for “prayed” and you see how silly that kind of logic can get. Unless there is good scientific evidence supporting “mushroom pills” helping with his problem, it’s not reasonable to assume that one has anything to do with the other.
To the people who seem to think that “natural” methods are better than scientifically tested procedures, there is zero reason to come to that conclusion when looking at the overall evidence. Yes, this treatment or something like it may be more helpful in some cases, but to say you would prefer it over chemotherapy despite knowing so little about it suggests a sad distrust of the medical community. If a “natural” method worked under scientific conditions and was relatively safe then it will be adopted. The “natural” methods rejected by science were usually rejected because they didn’t work (like homeopathic treatments) and/or they weren’t safe.
I know some people will come down with the “but the medical industry won’t sell anything they can’t patent” nonsense, but it’s complete bunk. Doctors will prescribe whatever works. I’ve been treated twice using treatments made from belladonna for two different things because it works for those treatments. The fact that it’s natural wasn’t part of the equation.
And finally, to the “it’s natural therefore it’s good for you” crowd, I say go chew on an all-natural poison arrow frog. ;-P
magkneetoe said: “Cancer is abhorrent. Sometimes I wonder if there are better ways to battle cancer, even to cure it, out there. How much money does the health care industry make from of cancer? A better question, how would they lose if they had a simple, cost effective cure for cancer. You wouldn’t need the business of expensive chemo or radiation or MRI’s or surgeries or etc that cancer brings. I couldn’t imagine that it would be possible for something as reprehensible as that to take place, but I can’t imagine half of the injustices of the world.”
The problem with that is that it would take a concerted effort on scientists all over the world to maintain a cover-up on cancer cures, and not one anywhere has made a peep in the years since they were discovered. This would require an unimaginable level of greed and evil that seems just silly when you realize that a cancer cure would provide unlimited revenue to the company that found it, since people will never stop getting cancer even if there’s a cure. I mean, we have cures for all sorts of things (like cataracts,) but that doesn’t mean that the problem vanishes.
The responses to this are amusing me. So many people saying they’d want Coley’s treatment in lieu of chemo or radiation. Now, I’m not a doctor…and I don’t play one on TV…but I’m quite sure it doesn’t work like that.
This DI article…while quite interesting…isn’t going to ‘allow’ you to choose this type of treament instead of chemo or radiation. You’d get whatever treatment was best suited for your specific type of cancer. Unless you’re footing the bill yourself and can find a doctor willing to treat you with something that would only provide negative effects…good luck.
Just like SWehrly said, these types of treatments don’t work on every type of cancer. Saying you’d choose Coley’s treatment instead of chemo or radiation is just silly. Saying treatments like Coley’s would be preferred or that you’d hope to be treated that way is one thing….saying you’d CHOOSE it over another treatment makes no sense.
I’ll take whatever treatment keeps me alive, if I am unlucky enough to end up with cancer…for some reason I don’t think the treatment will be my choice. If I do have a choice….I’m sure there would be a lot of risk involved in whichever choice I made. Bottom line….I’ll take whatever treatment the Doc recommends.
HiEv said: “The problem with that is that it would take a concerted effort on scientists all over the world to maintain a cover-up on cancer cures, and not one anywhere has made a peep in the years since they were discovered. This would require an unimaginable level of greed and evil that seems just silly when you realize that a cancer cure would provide unlimited revenue to the company that found it, since people will never stop getting cancer even if there’s a cure. I mean, we have cures for all sorts of things (like cataracts,) but that doesn’t mean that the problem vanishes.”
I’ll one up ya….what if we already know what CAUSES cancer? Would you get rid of that stuff? You’d think it would be done without hesitation, yet cigarettes are still legal. Why is that? The fat cats get fatter either way….the revenue from cigarettes and the more from cancer treatments. That’s big bucks.
It wouldn’t surprise me if there already was a cure for cancer. Let’s assume for a second there is and it was found in the US (makes it easy). How could they keep that hush hush? How do they keep the truth about JFK’s assasination quiet? Aliens?
The answer is, they don’t. Any scientist that comes out and says there is a cure for cancer and the US government is keeping it secret would be branded a lunatic and would never find work again. The best conspiracies don’t need cover ups….because the lie is so huge anyone that knows about it would never be taken seriously if they tried to get the word out.
The moon landing was fake
Alien aircraft are being reverse engineered at Area 51, The CIA had Kennedy killed, 9/11 was an inside job,
Elvis faked his death, A secret society runs the world, Larry King is actually a robot (with many software glitches).
Get my point? All of those could be true…but they would be soo big that even if I know FOR A FACT that one of them was true….nobody would believe me. Nobody would have to silence me, because I’d never be believed by anyone.
Many people claim there is a cure for cancer…many people claim perpetual motion has been discovered…are they taken seriously? Nope. Should they be? Possibly, but they never will be.
DI article. I’ve heard that 1 in 3 people will get a serious form on cancer during their lives, so many people are affected by it – no wonder there are so many comments!
My brother got acute APML lukaemia 5 years ago and he was treated with arsenic, not chemo and transfusions, we were so happy to see him out of hospital and in full remmission after 28 days. It was sad to see other guys his age (18, 19’ish) still in and out of hospital a year later. I know these things don’t always work for everyone, but all I can input on the conversation is check your options thoroughly. The doctor (head of oncology) didn’t think my brother would make it on that treatment – but he’s really had the best result possible.
Also this article reminded me a bit of how malaria get’s treated, a series of fevers kill off the bad stuff (not very scientific, but I’m struggling to remeber details).
I don’t believe that people should all rush to the idea that this idea is better than chemo or radiation. There’s no way to know that, and as it has been said, there are so many types of cancer that there will never be a magical “cure-all” for all of it, or that will work for everyone.
Do I think it’s possible that there’s a cure out there that’s being kept under wraps? Yes, but not very likely. I do believe that many practitioners of western medicine are unable, or unwilling, to look at alternatives to what they see as a “tried and true” treatment. I am also not stupid enough to believe that because something is deemed “natural” it is also perfectly safe. It’s just another way to look at things or treat things. Also, just because something does not have a lot of scientific studies to back it up does not mean that it is bunk. There is no scientific study to prove the existence of God, yet there are many people who believe there is one, in one form or another.
I believe it’s all about having an open mind.
HiEv: Thank you. You put my exact thoughts forward in a much more succint and pure way than I would have been able to. It’s absolutely ridiculous to try to claim that the entire medical profession is a bunch of money-hungry thugs who would rather see people debilitated than healthy.
Jeffrey93: I also agree completely with your statement, at least the first one. As to the second: The Moon landing DID happen, the 9/11 conpiracy theory requires such precision that it’s master-minds would have had to be robots or something, and perpetual motion is impossible in any environment containing anything at all. Conspiracy theorists are regarded as nut-jobs for one reason…. because they are. (Though, to be perfectly honest, I’ll spot you aliens– they probably do exist, it’s pretty much a mathematical guarantee– but I refuse to believe that {given the vast distances they would be forced to travel} they have any sort of direct effect on the collective human civilization, or any interaction with humans at all.) In any matter, this conspiracy mumbo-jumbo is so far off-topic that it can barely be presented as tangental, so let’s all let it go.
Streetbob80 said: “I just keep thinking about how kids are staying indoors so much nowadays and not playing outside as much. It seems to me that running around in the woods, catching frogs, and swimming in creeks would initially build up the immune system to later combat health issues in the future. I don’t know if this is how it all works, but it makes sense to me. And all of the parents that teach their children to not get dirty and wash their hands 20 times a day, just seems to me that they are preventing the immune system to develop into a healthy and strong combatant. Like I said, I may not be right, but thats the way I see it. Let nature do its job.”
Actually, all that exposure to sunlight you’re talking about LEADS to cancer. I’m not opposed to children playing outside (in fact, I’m all about it), but there are inherent risks in any situation. I know I’ve got to die sometime, so I don’t really mind getting sunburnt and dirty– it’s really all about choosing your poison (unless, of course, you get hit by a bus tomorrow–not a lot of choosing going on there).
Whoa, whoa whoa. That woman got cancer from getting her hand smashed?!?! You can get cancer from that?!?
Screw it, I’m going on a smoke break.
Did I have too much coffee today, or was the title of this post changed?
RichVR said: “Did I have too much coffee today, or was the title of this post changed?”
I think it did. Is our new writer trying to confuse us? Or add a little razzle-dazzle to impress the boss? Alan always applauds the addition of alliteration to an article.
Wow! I will ask my doctor what he thinks about immunotherapy next time I see him; he’s up on random bits of medical knowledge. However, I imagine there must be a better reason why immunotherapy isn’t used more widely… I’ll find out soon. There must be a serious risk somewhere… any doctors in the hou… I mean comment thread?
Oh! I hear of people claiming to create perpetual motion machines all the time (suprisingly alot of farmers), but it’s just not possible. The energy has to come from somewhere, and you never get out what you put in. Sorry I saw a bit about that in the comments and I’m in thermodynamics… had to say something. The US won’t even allow people to patent them any more because the inventor would cause aot of investors to lose all of their money.
Now, pie-petual motion is an interesting concept. It’s some of the best motivation to do work. mmmmpi
Streetbob80 said: “I just keep thinking about how kids are staying indoors so much nowadays and not playing outside as much. It seems to me that running around in the woods, catching frogs, and swimming in creeks would initially build up the immune system to later combat health issues in the future. I don’t know if this is how it all works, but it makes sense to me. And all of the parents that teach their children to not get dirty and wash their hands 20 times a day, just seems to me that they are preventing the immune system to develop into a healthy and strong combatant. Like I said, I may not be right, but thats the way I see it. Let nature do its job.”
I believe recent research has confirmed this. Moms who keep their houses super-clean or bathe their babies every day compromise the immune system of their children. Also, children who grow up with dogs or cats have stronger immune systems. Anti-bacterial soaps, as you’ve probably heard, are also a danger, as they destroy the good bacteria with the bad.
So what’s the philosophy behind not using a treatment you don’t understand? If a mysterious treatement works better than an understood treatement, why not go for the mysterious treatment?
My guess is that doctors like to know what’s going on with a treatment so as do know what other treatements they can or cannot give, like not giving someone asprin for a headache because they know the person could be allergic, or being careful what drugs or treatments are best not to mix.
Relying on a plan of universal understanding provides a common to compare anything, but using a treatement that you don’t know how it works invites a pandora of problems. Though in this case since the treatement is natural (your using a natural bacterium to induce a natural immune response), you’d think the doctors wouldn’t be concerned about bad mixing of treatements.
I can’t believe I’ve never heard this before. Thanks for the post!
There’s another interesting cancer treatment I was just reading about that doesn’t seem to be getting much press, and it’s an interesting approach that’s easy to explain. The treatment was developed at Duke University.
A standard chemotherapy drug is encapsulated within an innocuous chemical known as a liposome and injected into the bloodstream. The encapsulation keeps its contents from interacting with the body because it’s completely hidden inside. The lipsome is heat sensitive and will open up if the temperature rises to between 102ºF and 108ºF, and that’s where the second technology comes in.
Radio waves, close to microwave frequencies, are passed through the body from a wide angle and focused on the location in the body where the tumor resides. The energy level is chosen so that the temperature rises to the right range for the liposome to expose its payload, between 102ºF and 108ºF, which of course only happens at the sight of the tumor where the energy is focused.
The result is that the chemotherapy treatment is precisely targeted, and a much lower dose can be administered while still achieving the concentrations required at the site of the tumor. Yes it’s chemotherapy, but the side affects are greatly reduced. Currently, clinical trials are nearing completion for thermo-encapsulated doxorubicin, which they’re calling “Thermodox” in it’s encapsulated state.
It seems to me this approach would have lots of potential for any drug that would benefit from being targeted, like Coley’s Cancer-Killing Concoction. After all, Coley injected his solution “directly into the tumor mass”, right?
I guess “sight of the tumor” also makes sense, but I meant to say “site of the tumor”.
Touchy said: “So what’s the philosophy behind not using a treatment you don’t understand? If a mysterious treatement works better than an understood treatement, why not go for the mysterious treatment?”
I never understood that philosophy either, and you see it all the time in medicine. It’s considered “unscientific” for a doctor to proscribe a treatment when he doesn’t understand the mechanism, and if he proscribes it anyway he’s afraid to tell you that. That’s why there are so many different medical ways to say “I don’t know” that sound more impressive than “I don’t know”. Medical science always seems more vain than the other sciences (that’s ‘vain’ not ‘vein’ :)
But in the case of Coley he started with a real hypothesis which he tested scientifically and could then show unambiguously that it was effective in the same way he had predicted. Is it really necessary to know EXACTLY how it works? Do the pharmaceutical companies know exactly how all their drugs work? Even the ones that were discovered by accident, like Viagra?
I am still trying to figure out why exactly Coley’s Toxin was shoved aside for Chemo. Generally, when something “bigger and better” comes along the other treatments are still around. Like with prescriptions, when a newer one comes out, the others just go over the counter instead of being prescription. I just can’t see Coley’s being not used at all when a new treatment came out because that seems to be unheard of today. Where there other side effects that we have not been presented or was it still used and just not documented as much?
Floj said: “The US won’t even allow people to patent them any more because the inventor would cause a lot of investors to lose all of their money.
Is that the current policy, Floj? I thought that the USPTO simply refused to accept patent applications for such things unless accompanied by a real, working model. Which they’ll never get, of course….
Pie be unto you, my friend. :-)
@Touchy: I agree it’s weird – I’m pretty sure I’ve read a ton of medical literature on popular medicines (mainly, in fact, the little leaflets you get in boxes of medication) which admit that the exact method by which they work remains somewhat mysterious. It sounds like immunology pretty much just fell from favor…
I’m so glad I found this site! Impressive that Dr. Coley was so diligent in research!
As to the infections that killed the test subjects, it is important to note that there were no antibiotics available at the time of those tests, and that today, those patients would probably not have died, because the drugs exist to kill the overgrowth of Strep.
Davidlow said: “… It’s considered “unscientific” for a doctor to proscribe a treatment when he doesn’t understand the mechanism, and if he proscribes it anyway he’s afraid to tell you that. …”
Being picky: I believe you meant “prescribe” – to lay down in writing – rather than “proscribe” – to denounce, condemn, or prohibit.
To those of you complaining to those of us who have speculated that we might prefer Coley’s treatment to radiation/chemo on the grounds that (a) there must be a good reason and (b) there is no choice – the HMO/doctor chooses for you:
a) I qualified my answer with “I think”. When I am sick (thankfully rare), I like to know everything I can about what I have and the treatment. If I had cancer, I would do more research and consult with my doctor.
b) I live in Germany – no HMOs here :-) . I consult with my doctor. If my doctor doesn’t have time for that, I change doctors. I’ve learned the terminology, so it is not so hard.
City of Hope is not only a cancer hospital – they also do really amazing cancer research right in the same facility. My sister works there as a research scientist, where they are now using bionanotechnology to hopefully target cancer cells directly. You guys should do a Damn Interesting article about this work!
You can read more about the lab at City of Hope who works on this here:
http://www.cityofhope.org/Researchers/SmithSteven/SteveSmithLabHome.htm
My mother had bladder cancer several years ago and she was treated with tuberculosis “toxins” that she had to hold in her bladder for a prescribed amount of time. No one ever told me if it was an experimental treatment, I don’t think they told her that, either. It worked and they haven’t found anything amiss since. It sounded scary at the time, but the side effects were nothing compared to those of aggressive chemotherapy and radiation. I hope the medical community can get more comfortable with this kind of treatment. In that compromised position, and with my limited knowledge, I’d much rather have my immune system boosted than wiped out.
If we don’t understand how something works, we can only prescribe it based on empirical data and anecdotes, a practice that is dangerous under the best circumstances. Without knowing how the curative effect is reached at, there is no way to figure out what the side effects may be, and in many cases these side effects may be worse than the disease itself. It will also be indefensible in court if something bad does happen later on–adverse effects may take years to present themselves.
RichVR said: “Did I have too much coffee today, or was the title of this post changed?”
That might be the extremely misleading re-titling it got when posted on Digg: “A Cancer Cure Most Doctors Won’t Tell You About”. I guess it’s true that most doctors won’t tell you about it, probably because they don’t know about it. But there’s nothing in the article itself or original title that suggests any kind of medical conspiracy to keep Coley’s method secret, as implied from Digg. Just an interesting historical footnote in cancer research- which as the end of article hints, is probably going to be made irrelevant by more precisely researched immune-related treatments in the future. Whether or not Coley’s ideas were valid, it was still essentially a 19th century treatment which was never properly understood, never went through a modern drug certification process, and was never a single, consistent product in the first place. The fate of the Northwick Park Hospital drug trial volunteers in north London last year is worth considering- there are real risks to immunoactive treatments too.
I know a gentleman who has been cured of bladder cancer using a disabled bovine tb germ. We will never understand cancer until we realize that it originates from a stem cell, one that has been with us from our days as a fetus. Then, we actually have to be able to study embryonic stem cells without the murderous fools from the church standing stupidly in the path of science and human health, as they have done so very many times before. Check out Laird in a recent Nature Genetics, from USC, Laird claims that cancer is from an embryonic stem cell that was silenced in the fetus, then is reawakened later in life. Gostjeva,(MIT( Jan 2006 paper on bell shaped nuclei, stated that colon cancer stem cells and stem cells making a new fetal colon were identical, and imaged them. She arrived at same coclusion as Laird. She also discussed that cancer could also originate as an adult stem cell that is reverted to an embryonic state. Since here paper several genes have been identified that can make that transformation.
The expression of telomerase, and its upregulation of 70 growth genes, is the essence of both the fetus and cancer. Telomerase expression in cancer is all inclusive, pancancer, and also causes the downregulation of 147 genes that would cause a cell to be normal, and mature. (Blackburn, Ellizabeth)
Warburg noticed 80 years ago that a fetus and a cancer both run on glycolysis, now Sabet et al at UCSF claim that telomerase activation is the event that turns on glycolysis.
Oh war on cancer, where is thy song ,of victory,,,, after so long.
what does anyone know about this? I “stumbled upon” this story:
The University of Alberta Discovery
DCA is an odourless, colourless, inexpensive, relatively non-toxic, small molecule. And researchers at the University of Alberta believe it may soon be used as an effective treatment for many forms of cancer.
Dr. Evangelos Michelakis, a professor at the U of A Department of Medicine, has shown that dichloroacetate (DCA) causes regression in several cancers, including lung, breast, and brain tumors.
Michelakis and his colleagues, including post-doctoral fellow Dr. Sebastian Bonnet, have published the results of their research in the journal Cancer Cell.
Scientists and doctors have used DCA for decades to treat children with inborn errors of metabolism due to mitochondrial diseases. Mitochondria, the energy producing units in cells, have been connected with cancer since the 1930s, when researchers first noticed that these organelles dysfunction when cancer is present.
Until recently, researchers believed that cancer-affected mitochondria are permanently damaged and that this damage is the result, not the cause, of the cancer. But Michelakis, a cardiologist, questioned this belief and began testing DCA, which activates a critical mitochondrial enzyme, as a way to “revive” cancer-affected mitochondria.
The results astounded him.
Michelakis and his colleagues found that DCA normalized the mitochondrial function in many cancers, showing that their function was actively suppressed by the cancer but was not permanently damaged by it.
More importantly, they found that the normalization of mitochondrial function resulted in a significant decrease in tumor growth both in test tubes and in animal models. Also, they noted that DCA, unlike most currently used chemotherapies, did not have any effects on normal, non-cancerous tissues.
“I think DCA can be selective for cancer because it attacks a fundamental process in cancer development that is unique to cancer cells,” Michelakis said. “One of the really exciting things about this compound is that it might be able to treat many different forms of cancer”.
Another encouraging thing about DCA is that, being so small, it is easily absorbed in the body, and, after oral intake, it can reach areas in the body that other drugs cannot, making it possible to treat brain cancers, for example.
Also, because DCA has been used in both healthy people and sick patients with mitochondrial diseases, researchers already know that it is a relatively non-toxic molecule that can be immediately tested patients with cancer.
”The results are intriguing because they point to the critical role that mitochondria play: they impart a unique trait to cancer cells that can be exploited for cancer therapy”
Dario Alteri
Director University of Massachusetts Cancer Center
Investing in Research
The DCA compound is not patented and not owned by any pharmaceutical company, and, therefore, would likely be an inexpensive drug to administer, says Michelakis, the Canada Research Chair in Pulmonary Hypertension and Director of the Pulmonary Hypertension Program with Capital Health, one of Canada’s largest health authorities.
However, as DCA is not patented, Michelakis is concerned that it may be difficult to find funding from private investors to test DCA in clinical trials. He is grateful for the support he has already received from publicly funded agencies, such as the Canadian Institutes for Health Research (CIHR), and he is hopeful such support will continue and allow him to conduct clinical trials of DCA on cancer patients.
Michelakis’ research is currently funded by the CIHR, the Canada Foundation for Innovation, the Canada Research Chairs program, and the Alberta Heritage Foundation for Medical Research.
“This preliminary research is encouraging and offers hope to thousands of Canadians and all others around the world who are afflicted by cancer, as it accelerates our understanding of and action around targeted cancer treatments,” said Dr. Philip Branton, Scientific Director of the CIHR Institute of Cancer.
DCA and Cancer Patients
The University of Alberta’s DCA Research Team is set to launch clinical trials on humans in the spring of 2007 pending government approval. Knowing that thousands of cancer patients die weekly while waiting for a cure, Dr. Michelakis and his team are working at accelerated speed, condensing research that usually takes years into months. Fundraisers at the University of Alberta are determined to raise the money to allow this next phase of research to begin. Once Health Canada grants formal approval, the University of Alberta’s Research Team will begin testing DCA on patients living with cancer. Results with regards to the safety and efficacy of treatment should be known late this year.
“If there were a magic bullet, though, it might be something like dichloroacetate, or DCA…”
Newsweek, January 23, 2007
UPDATE January 23, 2007 – Investigators at the University of Alberta have recently reported that a drug previously used in humans for the treatment of rare disorders of metabolism is also able to cause tumor regression in a number of human cancers growing in animals. This drug, dichloroacetate (DCA), appears to suppress the growth of cancer cells without affecting normal cells, suggesting that it might not have the dramatic side effects of standard chemotherapies.
At this point, the University of Alberta, the Alberta Cancer Board and Capital Health do not condone or advise the use of dichloroacetate (DCA) in human beings for the treatment of cancer since no human beings have gone through clinical trials using DCA to treat cancer. However, the University of Alberta and the Alberta Cancer Board are committed to performing clinical trials in the immediate future in consultation with regulatory agencies such as Health Canada. We believe that because DCA has been used on human beings in Phase 1 and Phase 2 trials of metabolic diseases, the cancer clinical trials timeline for our research will be much shorter than usual.
Anyways, for those people who think that doctors and companies are hiding a cure to cancer. That is foolish. If a cure for cancer was found, they would release it as fast as possible. The person/company who found a cure to cancer would gain immediate fame, wealth, and power. The founder would het his/her money and fame, while the company would get immensly rich by being the one company that has the cure, and having a head start on selling the cure. They would sell the cure extremely quickly, while other cures like chemotherapy would be put on hold. Because of this, I can say that no one is hiding the cure to cancer.
I found this topic DI and like others I wonder why I never knew this before. also,
white_matter says:
Whoa, whoa whoa. That woman got cancer from getting her hand smashed?!?! You can get cancer from that?!?
Screw it, I’m going on a smoke break.
I too was amazed to read about that at first. There’s actually a term for when genetics are nudged or altered by environmental factors to produce a malignancy, but I can’t think of it presently. I think tissue damage alone can lead to cancer in rare cases too if a damaged cell (with damaged DNA) divides. I suppose all the variables fell into place for that poor girl and she got the big C while healing from her injury. As for the smoke break, why purposely increase your risk?
THANK YOU HiEv for supplying a little bit of reason to this discussion. I’d like to add a few points.
1.Drugs are not chosen or discarded due to lack of knowledge of their mechanism of action. (In fact there are numerous drugs on the market with poorly understood mechanisms of action- 1st one that comes to mind off the top of my head is the antiepileptic drug Keppra). They are chosen based on the results of large, randomized, double-blind, placebo controlled RESEARCH in which the therapeutic benefits and adverse effects are examined. There are many steps (referred to as phases) through which a new drug must pass in order to be approved, at least in the US. As many readers have pointed out, there is an enormous amount of ongoing research in the field of oncology. Much of this research involves attempts to better focus the treatment on the cancer cells and spare the rest of the body.
2.The idea that infection with various pathogens always leads to an improved immune system is simply bogus. Keep in mind that MANY cancers are actually caused by viruses (EBV, which causes mono, can cause lymphoma, HPV causes cervical cancer, HTLV causes leukemia, Hepatitis C can lead to hepatocellular carcinoma, etc. etc. etc.). HIV predisposes patients to lymphoma, kaposi’s sarcoma, etc. by destroying the immune system.
3.Please also keep in mind that there are reasons why chemotherapy and radiation are used. For example, childhood leukemia used to be a death sentence unless the patient had a spontaneous remission. Today, thanks to chemotherapy, over 80% of kids with acute lymphoblastic leukemia (the most common childhood form) are cured. Granted, there are side effects, both short- and long-term, but this is a great success story.
4.What criteria do people use to determine if a treatment is “natural” anyway. In my experience, this differentiation seems arbitrary. All medications are based on materials naturally found on earth. The VAST majority of medications are made from plant derivatives anyway. Cancer itself is natural, and nobody would claim that it’s a great thing.
5.The woman did not get cancer from getting her hand smashed. Cancer development requires damage to DNA leading to loss of genes that suppress tumor growth and/or induction of genes that promote uninhibited cell reproduction (proto-oncogenes). These genes are also being targeted in cancer research.
6.The idea that keeping a cancer cure from the public would somehow make money for people is insane. Hiding something like that would prevent the discoverer, manufacturer, etc from making infinite $$, without even going into the horrific ethics one would have to possess in order to keep a “magic bullet” from cancer patients.
Bottom line, cancer is a diverse group of diseases with diverse causes, and different types respond or fail to respond to different treatments. Progress has been made, but there is not and never will be a “magic bullet” that cures all cancer. The best way to continue with progress is through scientific investigation of potential therapies, which is currently happening all over the world.
westcliffer said: “…. As for the smoke break, why purposely increase your risk?”
’cause it’s fun.
smoking is fun
Know what else is fun? Not getting cancer *nods*
Modern medical scientists should divulge on understanding the behavior of the bacteria on the cancer cells.. This would make a great treatment to cancer patients aside from therapy and surgery.. Imagine how many lives will be saved from pain and treatment fees.. ^^
CravenMorhead said: “In my minor in biology I never heard this, and I took a number of genetic and microbial courses. I can’t even fathom the mechanism of this but what I think could be happening is that the caner cells don’t have the proper antigens on their membrane. They probably don’t have any at all considering how a cancerous cell develops. Usually they fly under the radar, they aren’t considered dangerous because they don’t have anything that the immune system considers bad on their membrane. Then comes the infection. The body is suddenly put under marshal law, while taking out the bacteria, which have a foreign and hostile antigens on their membrane, the macrophages start to consume the cancerous cells as well. The cancerous being unknown and possibly dangerous. Kill them all and let the body sort them out.
It is an interesting hypothesis, probably complete bullshit.
/
I have a major in biology and that is the hypothesis I believe is the most likely as well. And than there is the possibility, that cancer simply doesnt like cytokines. Those cells have corrupted many of the proper pathways in favor of excess growth, it may very well be that some chemical signals which are flooding the body during illness, are lethal to cancer cells.
One big problem with the conspiracy theory is that whoever starts using the miracle cure first would promptly corner the entire market, and make a fortune. the investors, other companies etc would hurriedly chase after the bonanza.
It’s not just “big bucks,” it’s also freedom of choice. And freedom of choice means the right to make stupid decisions for yourself, including smoking. Yes, the tobacco industry has committed numerous heinous acts to keep their product on the market, but if we want a truly free society then people should have the right to poison themselves as long as they’re fully aware of the damage they’d be doing to themselves and capable of making a mature decision.
Just to be clear about my position, I don’t drink, smoke, or do any drugs except for the occasional aspirin unless prescribed by a doctor. However, I think it should be every mature adult’s right to do those things as long as they’re aware of the dangers and don’t endanger others while doing them.
Then you must have a pretty low opinion of humanity. Putting aside the silliness of treating the wide variety of cancers as though they’re all the same, how could anyone in good conscience keep such a thing a secret? How can you imagine some researcher who finally manages to discover this holy grail of medicine and then decides to cover it up, even though it won’t really benefit him or his company to do so? Heck, covering it up just means that they’d lose the rights to the cure if some other group came up with the same cure! It’s not like they could have a public squabble over the rights, because then they’d have to admit sitting on a cure for cancer.
I think some people don’t really consider how ridiculous some of these conspiracies are because their analysis of the situation is so shallow and unrealistic.
Uh… What makes you think that the truth isn’t out? J.F.K. was killed by Lee Harvey Oswald and no aliens have visited us yet. The vast preponderance of the evidence seems to make those the most probable answers.
Unless they have one simple thing: evidence. Want to prove aliens? Bring out a real alien or some clearly alien technology. Want to prove you cured cancer? Produce the cancer cure. Once you do that, POOF!, no more cover-up is possible.
The reason why the conspiracy nuts are treated as nuts is because they don’t have any good evidence, they just draw unlikely conclusions from bits and pieces of information, ignoring rational explanations, and are left without any real proof that their conclusion it better than the far more likely conclusion that most everyone else has reached.
First of all, most of those can’t be true unless you ignore the vast preponderance of the evidence against them. For example, there are artifacts on the moon that can be seen from Earth, which proves that we landed on the moon. Furthermore, as I pointed out above, people would believe you if only you had good evidence for what you were saying.
It’s amusing about how you talk about “fat cats” and “big bucks” when talking about the tobacco industry, but you seem utterly blind to how many people would be willing to finance a cure for cancer because they’d see huge dollar signs when they’d think about the profit and good PR to be made. Even if we cured cancer, it’s not like cancer would go away, you know? There’d always be new cases occurring, thus it would continue to turn a profit indefinitely.
Furthermore, take a look a polio. Do you think the medical industry would have made more money from hiding the vaccine and treating polio victims forever or by releasing the vaccine and nearly eradicating it from the planet? Obviously they’d make more money from the former, but I bet you can’t find even one person who suggested such a wretched and selfish course of action.
Unless they simply provide some good evidence, which shouldn’t be too hard if they actually have such a cure. The fact that they never produce such a cure is evidence that they’re either delusional, mistaken, or flat out lying.
Your whole argument hinges on the idea that these people can only produce hearsay, and totally ignoring the possibility of producing objective evidence for their claims.
Most points there are good, sound ones.
RE #5: The development of certain malignancies arising out of a site of trauma is, as one scientific journal article put it, “unusual but not rare.” Do a quick search to learn more.
http://www.google.com/search?hl=en&q=malignancy+arising+from+site+of+trauma&btnG=Google+Search
And here’s one type of cancer that can arise in such an instance:
http://en.wikipedia.org/wiki/Marjolin's_ulcer
p.s. RE #1: It is harder to get things through the FDA, today, without documentation of mech. of action… (something that the design of a phase I clinical trial should tease out or confirm).
Thanks for the good article DI folks.
well its nice shairing…..
http://www.eyecatchypics.com
HiEv, you are well respected around the forums and I enjoy your views, mosly because they conflict with mine. However, you obviously do not believe in anything unless there is a good amount of evidence to support it. I agree partially. However, I also find it to be just as irresponsible of you not to question the possibility of alterior motives or possible cover ups. These are things we have seen throughout history… to simply deny everything… and believe what is shoveled to you is just as irrational. Sometimes a good amount of skepticism is healthy. I quoted this because, if you like evidence… Oswald was not the shooter. I mean.. why lock up all the evidence, away from the public view for over 100 years… they must be hiding something. Also the CIA did issue a statement that a possible conspiracy was enacted.
It makes one wonder why Cancer still rules supreme in the world of human afflictions. Is it disease, politics or finance that gives cancer the winning edge?
My grandmother (born in the late 1890’s) used to always say: It’s a good thing if you get a bad cold in the late summer or early autumn. It boosts your immune system and you won’t get the flu in the spring!
Maybe she was onto something?
Who is currently researching the possibility (and methadology) of “stimulating the body’s immunity as a whole?”
THIS DAMN INTERESTING!
I am a doctor and I never read anywhere about this treatment. This is extremely important. Millions of people are dying of cancer in the third world because they cannot afford chemo. This type of treatment can give them an affordable chance of survival.
I think this type of immunomodulation can be refined and the type of cancer that best respond to it can be targeted effectively and economically. There are millions of bacterias that can be tested.
As someone mentioned earlier, some bacteria and viruses trigger cancer, because they induce changes in the cell, but apparently, from this article it seems that other types of bacteria helps the immune system. I can imagine it is the same thing as the good gut bacterias that helps in digestion and the bad gut bacterias that makes you sick.
I think Radiatidon would be interested in my opinion on the harm from electrical radiation:
http://www.biotele.com/EMI.htm
Can you give me the name of your doctor? I am looking into alternative treatment, preferably Coley’s Toxins, and am having a terrible time finding a single doctor who will administer it!
Thanks!
Most medicine is black magic combined with a complex appearance to reassure people. That in and of itself potentially cures many symptoms. It is not known how many antidepressants work, and malpractice lawsuits are correlated to bedside manner, not results. The litany of embarrassing facts is Piled Higher and Deeper, but it’s all we have.
Let’s not mention HMO “risk managers” that weight the cost of exam, treatment with the patient’s life versus profit. These gods have it easy because they never have to bother with meeting people face-to-face who’s coverage / claims they’ve denied.
Damn interesting, and actually, as Robert Smith and perhaps others pointed research using “infection” to treat cancer composes a promising lead. I strongly disagree with his point about stem cell research however, it can still be privately funded, get public funding in some states let alone the rest of the world. Furthermore treatments for cancer based on these general ideas already proceeds apace.
I believe, along with others, that with sufficient bio-informatics analysis techniques we’ll be able to develop targeted therapies which address the specific genetic abnormality of every different type of cancer. We have a ways to go yet to reach that point, but I’m sure the day will come. We’d be better off spending money working on these therapies rather than embryonic stem cell research which promise the world but are actually a long way off from delivering. Don’t get me wrong, stem cells are exciting as hell and promising, but all the controversy surrounding it has unfortunately lead people to believe that stem cells are some sort of cure-all – if only the nefarious religious people would get out of the way. Far, far from it, sadly. I wish that were the case!
Here you can find observation data gathered by Canadian Clinic – Medicor Cancer
Centres durning DCA therapy:
http://www.medicorcancer.com/DCAtherapyData.html
and here are 4 cases treated with DCA:
http://forums.cancer.vc/respiratory-thoracic/158-case-study-1-mesothelioma-new-post.html
http://forums.cancer.vc/skin/157-case-study-2-melanoma-brain-metastases-new-post.html
http://www.medicorcancer.com/DCA-CaseStudy3.html
http://www.medicorcancer.com/DCA-CaseStudy4.html
here’s an lymphoma remission story using DCA B1 vitamine protocol:
http://forums.cancer.vc/hematologic-blood/54-ds-lymphoma-story.html
and here are other remissions:
http://forums.cancer.vc/hematologic-blood/62-case-non-hodgkins-lymphoma-apparent-complete-remission.html
http://forums.cancer.vc/genitourinary-cancer/48-wim-huppes-prostate-cancer-story.html
http://forums.cancer.vc/genitourinary-cancer/55-ps-kidney-cancer-story.html
http://forums.cancer.vc/lung-cancer/56-tom-mcghees-nearly-full-remission-non-small-cell-lung-cancer.html
http://forums.cancer.vc/lung-cancer/63-lung-cancer-remission-dca.html
http://forums.cancer.vc/digestive-gastrointestinal/47-colo-rectal-cancer-story.html
http://forums.cancer.vc/digestive-gastrointestinal/57-bills-bile-duct-cancer-story.html
http://forums.cancer.vc/digestive-gastrointestinal/81-gastro-intestinal-stromal-tumors-dca.html
for more info on alternative therapies and dca see http://forums.cancer.vc and for additional info see http://puredca.com and http://cancer.vc
Interesting article
I am an oncology nurse. I administer a variety chemotherapy agents, all of which have been vetted in large clinical trials. I am not aware of any large trials of these toxins, but I did find a link from the American Cancer Society:
http://www.cancer.org/docroot/ETO/content/ETO_5_3X_Coley_Toxins.asp
My hope is that one day that chemotherapy agents will be discarded as the state of the art in treatment improves.
Perhaps older treatments such as coleys toxins should be revisited and given scientific scrutiny. For the sake of my patients and their families, I hope and pray that we beat this scourge on mankind
You can cure yourself with Quercetin + Vitamin C. 2 supplements that cost only $10 and can be bought from many vitamin stores. Just a tip.
see:
http://www.treat-cancer.info/
To that #92 schang1984: Just no.
DI Article but I would have to see some more trials done and a bit more research into this method to be 100% sold.
Food for thought: Would it be possible (if the cancer was diagnosed early enough) to attenpt hightening the immune system using “Coley’s Toxins” and see if there is any slowing or shrinking of the cancer and then if there is no change start on radiotherapy and chemotherapy treatments? Possible early trial methods?
Possible later trial methods!!?? (I would hope that the first trials would not go straight into this without further reaseach).
I heard about this some years ago but didn’t realise the
truth behind what I’d heard, does it not make sense when smallpox was cured using cowpox virus also dead flu viruses Are used to create vaccines for new strains of flu and what about penicillin, a natural fungus used to kill
Germs and other harmful bacteria! ‘who’s idea was first
In all these discoveries? “It matters not”, what is important is the fact that these discoveries were made
The only sad mistake by William Coley apart from cutting open the poor girl was ‘and assuming I’m correct in this’ was that he used live viruses on his human guinea pig patients rather than Dead ones? as they do researching flu vaccines. Btw Just a thought rather than test cancers in a Petri dish in a clinically clean lab why don’t researchers expose cancer cells outside the labs in the corridors to see what viruses attacked them, how they reacted and what chemical changes may take place, who knows what may come of it? After all “everything” in life has its Achilles heel. I’m not a scientist so if the
Suggestion is heavily flawed or sounds stupid I apologise.
Dr. Coley observed nature and implemented a very interesting method to stimulate the immune system as the ultimate cancer cure. Even though this treatment was effectively regulated out of reach in 1963 by declaring it an unapproved drug, there are many ways to support the immune system and accomplish the same healing results. Here is one that has proven very effective:
On the 5-21-2013 episode of the Robert Scott Bell health radio program (show notes here; click the link in the first sentence for the audio: http://www.robertscottbell.com/blog/chris-barr-reversing-cancer-w-food-grown-selenium-chromium-silicon-b6-probiotics-liam-scheff-on-peak-oil-secrets-nuclear-saud-more) (other RSB show archives here: http://radio.naturalnews.com/Archive-RobertScottBell.asp), nutritional researcher/historian Christopher Barr discusses his cancer protocol. At 51:35 he says to use the following four times daily:
5 selenium from Innate Response
3 silica from Alta Health
1 GTF chromium from Innate Response
1 cup of Can-Gest Tea from Alta Health
Add B6 and probiotics for more support.
One elderly man refused to follow the full protocol or to quit his unhealthy habits (smokes like a chimney); he only takes the selenium. Despite this, he has halted tumor activity and is still thriving years later.
Here are more details about the protocol components:
Selenium is very protective against toxins, including radiation. I’ve heard reference to a medical study where 19,000 people were monitored over a period of 17 years; half were given selenium. They had practically zero cancer, while the other half had a typical 30% or so cancer rate. Per Chris Barr on the Sunday, 11-17-2013 episode of the RSB show (http://www.gcnlive.com/CMS/index.php/archivespage?showCode=36) about 39 minutes into the second hour he says that Dr. Joe Vincent researched all the forms of selenium on the market and found that the Innate Response Selenium is twice as bioavailable as the next best. It contains other whole food and botanical ingredients plus probiotics and enzymes for better absorption, and is 100-300 times more bioavailable than seleno-methionine. The “bad” synthetic form is sodium-selonite. The Japanese get 300-600 mcg per day in their diet. You may take over 1000 mcg per day if dealing with cancer or lots of radiation; otherwise, it sounds like 400-600 mcg is reasonable. Another cancer researcher concluded the best form of selenium is seleno-selenium. It’s very inexpensive (http://www.swansonvitamins.com/healthy-origins-seleno-excell-selenium-200-mcg-180-caps). This is the trade-marked SelenoExcell (as High Selenium Yeast) food form of selenium by Healthy Origins. http://selenoexcell.com/faq says that it is not just seleno-methionine but also other forms such as seleno-cysteine. The Innate Response selenium contains complimentary ingredients such as enzymes and probiotics that should make it very bioavailable. You can get selenium in Brazil nuts and sunflower seeds as well.
Silica (silicon) prevents the spread of cancer by building up collagen. You find silica in shiny vegetables such as cucumbers or bell peppers, or better yet go with a quality food grown source such as from Alta Health or a liquid form from Orgono Silica (http://www.orgonosilica.com).
Learn much more about all that silica does for you: On a 40 minute segment of the June 23, 2011 episode of the Robert Scott Bell internet radio show (http://radio.naturalnews.com/download.asp?fileid=321), at the 1:18:40 minute mark, Christopher Barr states that supplementation with a whole food form of silicon is more important for bones and ligaments than calcium. Key points mentioned:
>> a massive study showed > 800mg daily intake of calcium causes more bone fractures because it hinders silicon absorption; most people get enough calcium via diet
>> silicon rebuilds collagen, cartilage, ligaments, bones, and strengthens joints
>> the “silicon challenge” is recommended for knee, joint, hip. lower back, and shoulder injuries: 3 tablets of Alta Health Silca, 4x daily for 3 weeks
>> silicon is found in unprocessed whole grains and dark leafy greens, but processed foods, fertilizers, and soil depletion have led to deficiencies
>> a whole food vegetable form of silicon is needed; ground up silicon is not bioavailable, and massive amounts of horsetail contains a component that weakens muscles
>> silicon also has benefits besides bone health:
>>> silicon maintains strength and elasticity of arteries (and vitamin K2, found in cheese and natto, directs calcium to bones rather than allowing arterial calcification)
>>> soft fingernails that break easily and grow poorly reflects the status of bones and arteries
>>> silicon helps prevent Alzheimer’s disease by binding with aluminum, thus preventing collection in the brain
>>> cancer metastasizes by breaking down collagen, thus, silicon helps stop cancer from spreading
Glucose Tolerance Factor (GTF) chromium is an important mineral – a deficiency WILL cause diabetes, as discovered by NIH scientists in 1959. Modern agricultural practices have left a huge void of essential trace minerals in our diet, and the lack of GTF chromium is very apparent with one fourth of the nation diabetic or pre-diabetic. Chromium protects your chromosomes, but the right form is essential. Chromium pincolinate damages kidney and liver cells because it drives the chromium inside the cells rather than on the surface where it assists insulin to transport glucose into the cell. A good rule of thumb is to take 100 mcg of GTF chromium three times daily. Search the Robert Scott Bell show archives (http://radio.naturalnews.com/Archive-RobertScottBell.asp) for chromium to hear Chris Barr expound on the topic; here is a recent episode: http://www.robertscottbell.com/blog/princeton-student-vaccine-guinea-pigs-chris-barr-diabetes-defeat-omsj-beats-hiv-fukushima-fear-fda-transfats-selenium-solution-more/ (skip to about 9 minutes into the second hour).
The Can-Gest tea, B6, and probiotics round out the protocol by supporting the gut health which is where most of the immune system resides.
Just as Dr. William Coley discovered so many years ago, we are still learning to support our immune system and trust it to maintain our health.
Coleys toxins cost next to nothing to cure peeps so they use chemo for the monetary injection
I dated his granddaughter years ago, and she told me she was descended from a famous doc. 20 years later I heard about him on people’s pharmacy, and I was hey why hasn’t this been followed up? But looks like his results were not replicated…
Based on the article, Coley toxins uses the body’s immune system to fight the cancer/disease which is similar to traditional chinese medicine to boost body immunity rather than using anti-biotics. Due to the lack of understanding of such treatment, its a touch-and-go or 50/50 chance of full recovery method. I have visited the cancer ward where patients are subjected to chemo, the place is bleak.
I myself would prefer the Coley treatment if it ever comes to that. Either became healthy or come to an abrupt end which is less hassle rather than ‘hanging on’ and cost my family a fortune treating me.
Thanks for the article, its an eye opener.