© 2013 All Rights Reserved. Do not distribute or repurpose this work without written permission from the copyright holder(s).
Printed from https://www.damninteresting.com/the-isle-of-doctor-seaborg/
It was the summer of 1936 when Ernest Lawrence, the inventor of the atom-smashing cyclotron, received a visit from Emilio Segrè, a scientific colleague from Italy. Segrè explained that he had come all the way to America to ask a very small favor: He wondered whether Lawrence would part with a few strips of thin metal from an old cyclotron unit. Dr. Lawrence was happy to oblige; as far as he was concerned, the stuff Segrè sought was mere radioactive trash. He sealed some scraps of the foil in an envelope and mailed it to Segrè’s lab in Sicily. Unbeknownst to Lawrence, Segrè was on a surreptitious scientific errand.
At that time, the majority of chemical elements had been isolated and added to the periodic table, yet there was an unsightly hole where an element with 43 protons ought to be. Elements with 42 and 44 protons—42molybdenum and 44ruthenium respectively—had been isolated decades earlier, but element 43 was yet to be seen. Considerable accolades awaited whichever scientist could isolate the elusive element, so chemists worldwide were scanning through tons of ores with their spectroscopes, watching for the anticipated pattern.
Upon receiving Dr. Lawrence’s radioactive mail back in Italy, Segrè and his colleague Carlo Perrier subjected the strips of molybdenum foil to a carefully choreographed succession of Bunsen burners, salts, chemicals, and acids. The resulting precipitate confirmed their hypothesis: The radiation in Lawrence’s cyclotron had converted a few 42molybdenum atoms into element 43, and one ten-billionth of a gram of the stuff now sat in the bottom of their beaker. They dubbed their plundered discovery “technetium” for the Greek word technetos, meaning “artificial.” It was considered to be the first element made by man rather than nature, and its “short” half-life—anywhere from a few nanoseconds to a few million years depending on the isotope—was the reason there’s negligible naturally-occurring technetium left on modern Earth.
In the years since this discovery, scientists have employed increasingly sophisticated apparatuses to bang particles together to create and isolate increasingly heavy never-before-seen elements, an effort which continues even today. Most of the obese nuclei beyond 92uranium are too unstable to stay assembled for more than a moment, to the extent that it makes one wonder why researchers expend such time, effort, and expense to fabricate these fickle fragments of matter. But according to our current understanding of quantum mechanics, if we can pack enough protons and neutrons into these husky nuclei we may encounter something astonishing.

In the 1950s and 60s scientists worldwide were employing nuclear reactors, atom smashers, and particle accelerators to combine subatomic particles into heavier and heavier elements. It seemed that all atoms heavier than 82lead or 83bismuth were inherently unstable, and that packing on more protons and neutrons always shortened the atoms’ existence. As these progressively heavier synthesized atoms’ half-lives diminished from years to days to hours to seconds, the prevailing assumption among researchers was that science was approaching the end of the elemental road. Given the poor return on investment, excitement surrounding the synthesis of new elements began to wane. It seemed that the Nobel Foundation had long since given away their last prize for the discovery of a new chemical element; atoms that decompose within milliseconds were just not very useful or interesting.
But in the late 1960s, a comprehensively successful chemist named Glenn T. Seaborg made a bold prediction: despite the predominant view to the contrary, he asserted that there are likely to be some “superheavy” elements with very stable nuclei that had never before been seen by man. He was singularly qualified to make such a deduction, having personally discovered or co-discovered nine elements already. Later he would be credited with his tenth elemental co-discovery, his honorarily eponymous “seaborgium”. He had also worked on the Manhattan Project, advised several US presidents on nuclear policy, and acted as chairman of the United States Atomic Energy Commission from 1961 to 1971.
Seaborg’s insight was based on his thorough understanding of the nuclear shell model, which is one of science’s most accurate models of how the stuff of atomic nuclei might be organized. The model describes a system where the particles of the nucleus organize themselves into structures of progressively larger nested “shells”, each made up entirely of protons or neutrons. At atomic scales the strong nuclear force binds the nucleon particles of the nucleus together while the electrostatic force simultaneously presses them apart.
The strong force easily dominates petite nuclei such as 3lithium, keeping the nucleons in strict bondage. But in beefier elements on the periodic table such as 85astatine-210 (85 protons and 125 neutrons), the nuclei start to become girthy enough that mere attraction is insufficient to bind the bits together indefinitely. For these unstable atoms, it is only a matter of time until the struggle between attraction and repulsion results in a sudden discharge of nuclear material. This radioactive decay releases considerable energy as radiation, and reduces the original atom to a lighter element. The assorted ejecta may form into other lighter atoms and/or fly off as loose subatomic bits. For instance, when 89actinium decays in nature it can produce the lighter element 87francium. Francium tends to surrender to decay rather rapidly—usually within an hour—to produce atoms of 85astatine, 88radium, or 86radon, each of which further decays into other atoms. Francium’s abbreviated half-life means that only about 30 grams of of the stuff are present in the Earth’s crust at any given time. If one attempted to assemble some francium atoms together to observe the properties of this metal, any sample large enough to see would instantly vaporize due to the heat of spontaneous fission, and all unprotected biological observers would promptly perish.

Owing to atomic decay in heavy nuclei, about the heaviest atom one is likely to encounter here on Earth is 92uranium. Essentially all atoms with heavier nuclei have fallen apart over the past few billion years. This battle between attractive and repelling forces would seem to suggest that the life expectancy of an atom is inversely proportional to its obesity. In general, this is roughly true. Some heavier nuclei, however, deviate from the pattern and outlast their daintier cousins by a considerable margin. The aforementioned nuclear shell model ascribes this to the fact that these atomic outliers have a “magic number” of particles in their nuclei. When a nucleus has all of its proton shells or neutron shells loaded to full capacity, the layers can align so well that each shell can spoon intimately with its attractive neighbors, forming a more compact sphere that fits well within the strong nuclear force’s area of influence. When all shells of both nucleon types have a full complement, the layers snuggle spectacularly, and the tightly bound result is known as “doubly magic”. One example is 82lead-208, which along with other double-magic atoms will loiter around the universe for a very long time indeed.
Seaborg’s stimulating proposition was that the steady decline in cohesion at the end of the periodic table may not be a one-way dive into the stability abyss. There ought to be, he suggested, an “island of stability” where certain superheavy isotopes have enough nucleons of the right types to fill all of their shells and become magic or doubly-magic. These never-before-seen elements would possess sturdy, comparatively long-lasting nuclei. Presumably, some of these exotic isotopes would even be stable. Considering the flighty nature of the heaviest atoms yet wrought by man, Seaborg’s claim was counterintuitive at best, but the subsequent flurry of calculations indicated that he was almost certainly correct.
The obvious challenge to the island of stability hypothesis is to ask why we have not yet encountered any of these stable superheavy atoms in nature. If they indeed exist, they ought to be present on Earth in observable quantities like all of the other long-lived elements. The answer seems to be that the universe’s atom fabricators—stars and supernovae—don’t tend to create the conditions necessary to produce superheavy elements. The immense heat and pressure at the center of a large star is adept at fusing the universe’s primordial hydrogen, helium, and lithium into progressively heavier atoms. But once the star reaches the point in its life where it is producing atoms of 28nickel it begins to spend more energy than it gains from each fusion. Some stars can cook up atoms as heavy as 83bismuth before they are completely exhausted. Once the star is back in bismuth, it clenches rapidly, producing high-pressure internal shock waves that can spawn 92uranium, 94plutonium, and #%$!pandemonium. The star then explodes, scattering its astronomical atomic abundance into the cosmos. The heaviest natural elements are now known to be the product of neutron stars—giant stars which have burnt out and collapsed into spheres of pure neutrons. When two of these stars collide, some of their mass is ejected, and various heavy nuclei can coalesce from this soup of naked neutrons.
This galactic chemical evolution is the source of all of the natural heavy atoms we humans know and enjoy today. As famed astrophysicist Carl Sagan was fond of saying, “We are made of star stuff.” But despite stars’ best efforts to manufacture heavier atoms, today’s universe is still essentially 90% hydrogen and 10% helium, with the rest of the matter in the universe amounting to a rounding error. Humans, of course, then picked up where stars left off by synthesizing atoms that stellar fusion and supernovae are incapable of producing. As of this writing (January 2013) the heaviest element yet made by man is element 118, known by the temporary name “ununoctium”. In 2005 Russian and American researchers working in tandem produced several atoms of element 118 by colliding 98californium-249 atoms with 20calcium-48 ions. The 118ununoctium-294 nuclei had a half-life of less than a millisecond, but this was longer than would be predicted in a non-island theoretical framework, which further reinforces the island of stability hypothesis.
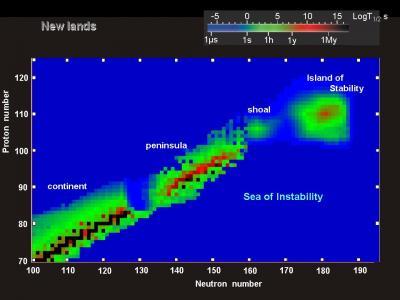
If atomic explorers ever do manage to slosh up the beach onto the island of stability, it is impossible to predict precisely what scientific novelties they will find there. Although chemists can make some guesses regarding the properties of stable and semistable superheavy elements based on the existing patterns in the periodic table, we cannot know whether or not these undiscovered atoms are useful or bizarre until science forges a few. Interestingly, the heaviest isotopes physicists have managed to synthesize so far don’t behave quite like science’s best current models predict, so stable superheavy nuclei are likely to be full of surprises. Chemists cannot even predict with any certainty whether these materials will exist as gases, liquids, or solids at room temperature.
The essence of chemistry—the basic reason that any chemical reacts with any other—is that atoms store their electrons in nested “shells” surrounding the nucleus, each of which can hold a limited quantity of electrons. Atoms desperately want their exposed outermost electron shell to be filled, and as they bump against other atoms they jettison, share, or burgle electrons in an effort to accomplish this. These transactions can be cooperative or competitive, resulting in peaceful or violent reactions. In the early days of elemental chemistry, the atoms in the first few rows of the periodic table were found to fill their outer shells in predictable patterns, producing somewhat predictable chemical reactions. But some oddities emerged as some heavier elements such as the lanthanides began to be discovered, consequently confounding the predictive models and forcing them to be revised. It is quite possible that the exotic island of stability elements will similarly diverge from expectations.
Dr. Seaborg’s original models suggested that we would find magic superheavy semistable nuclei in atoms of 114flerovium-298 and 120unbinilium-304, and doubly-magic nuclei in 126unbihexium-310. Subsequent discoveries in physics, however, show that such enormous nuclei would become deformed, thereby probably shifting the magic and double-magic quantities. Only with further experimentation can science be certain.
It is difficult to predict exactly how heavy nuclei can get even with doubly-magic nucleon shells. Famed physicist Richard Feynman allegedly suggested that element 137 may be the heaviest electromagnetically neutral element that can possibly exist in our universe since an atom with 138 electrons would require that the innermost electrons move faster than the speed of light. For this reason, the yet-to-be-synthesized element 137 is often referred to as “feynmanium.” Modern physicists using more sophisticated computations estimate that this limit may be nearer to element 173. Even in spite of this, physicists are not convinced that element 173 spells the end of the periodic table. Nature, as they say, finds a way. In fact, if one wishes to be particularly pedantic, one could point out that neutron stars are technically enormous atoms with preposterous atomic weights.

By the time he died in 1999, Dr. Seaborg had spent 30 years of his chemistry career attempting to traverse the treacherous channel between the known elements and the elusive island of stability. A year before his death, in 1998, researchers in Russia managed to synthesize atoms of 114flerovium-289 by crashing 94plutonium-244 into ions of 20calcium-48. Unfortunately these fused atoms were nine neutrons short of the doubly-magic 114flerovium-298, so they decayed rapidly. But Dr. Seaborg lived at least long enough to see atomic explorers get within sight of the shell-strewn “shores” of the island. Cramming in enough neutrons remains as the primary problem to solve in synthesizing stable superheavy elements.
If science ever does succeed in concocting these extraordinary materials, the atoms will certainly be expensive to produce in substantial quantities. If the new elements exhibit profitable properties, science will likely find a way to mass-produce them à la Manhattan Project plutonium. But even if they prove pedestrian, these unusual atoms would still represent a scientific first, and their study would greatly advance our knowledge of the building blocks of the universe. Indeed, perhaps there is a solid 79gold Nobel or two still on the table. Regardless, unless there is some alien civilization more advanced than our own that has beaten us to it, humanity may be on the cusp of creating elements unlike anything the universe has ever seen.
© 2013 All Rights Reserved. Do not distribute or repurpose this work without written permission from the copyright holder(s).
Printed from https://www.damninteresting.com/the-isle-of-doctor-seaborg/
Since you enjoyed our work enough to print it out, and read it clear to the end, would you consider donating a few dollars at https://www.damninteresting.com/donate ?
“92uranium, 94plutonium, and #%$!pandemonium” genius! :)
Excellent read as always Alan!
While Dr. Seaborg was alive when element 114 (flerovium) was first created, unfortunately he had had a stroke just two months earlier and never recovered to see the discovery.
Also, you wrote, “As of this writing (January 2013) the heaviest element yet made by man is element 118, known by the temporary name ‘ununoctium’.” That may be stretching things a bit, as in 2011 the IUPAC (International Union of Pure and Applied Chemistry) said, “The three events reported for the Z = 118 isotope have very good internal redundancy but with no anchor to known nuclei do not satisfy the criteria for discovery”. In other words, maybe they made them, but there’s not enough evidence yet to be sure. The report on element 117 are currently pending review, but 116 has been accepted and named (livermorium). It would be better to say 118 is the heaviest element reported to have been created.
There’s an excellent, if slightly out of date, video by NOVA Science Now that helps explain a lot of the physics involved:
Island of Stability (9/3/’06 – click the “Launch Video” button there)
Thanks for the article, looking forward to more!
Great article. I second the comment about the use of “pandemonium.”
Mr Segrè travelled all the way from Sicily to America, to get his colleague Mr Lawrence to post him something in the mail. It was only later that the moderate inefficiency of this process occurred to him.
Thanks for another great read, Alan!
I too grinned at the #%$!pandemonium reference…
Got one nitpick and one amplification for ya…
While the francium nucleus does indeed break up, “spontaneous fission” (SF) usually results in fragments larger than alpha particles; francium is not known to undergo SF.
G. Harry Stine, in a guest editorial for [i]Analog[/i], noted that the stability of atoms with Z greater than ~174 seems to be impossible. A few such atoms have been formed in collisions in accelerators, but they change Z by a unique method: the electric-field energy is so large near the nucleus that spontaneous pair production happens. The electron that is formed falls into the nucleus and neutralizes a proton, forcing Z down by one!
(The new nucleus is still highly unstable, so it decays rapidly, but by more conventional means such as SF.)
But what is the atomic number of Unobtanium?
For mysterious reasons, that number is unobtainable.
“Francium tends to surrender to decay rather rapidly”. Excellent.
Damn good article :)
The pandemonium line is hilarious. Way to go! The whole article is wonderful.
10q.
This was the first article I read on the first day I found this site, I couldn’t sign up and sign in fast enough. Hilarious, exceptionally well written and damn true to form, damn interesting. <3 good things
Thank you HiEv for the link to the rather cool Nova video. This was an awesome read. Learn something new everyday!
OK. So I stumbled on this article and though I had to clarify some of the terminology, I am fascinated! What this means in my uneducated brain is, they are close to creating (through the chemistry of combining elements) either a big bang or a black hole. The absorbtion of the mass with the super heavy elements or the big bang with the realease of energy through the seperation of elements? Essential also, doesn’t this have baring on time travel and astro physics? If you are dealing with elements that move faster than light. . . . . a) how could we ever see them? and b) wouldn’t that create the ability to travel through time to realise them? . . . . .I suddenly have a headache! Thanks for the fascinating article! I need to go meditate!
All that I can say is that I can’t comprehend the majority of this article.
Still lost.
And still lost.
I’m back.
I tried again, and I am still lost.
Yeah. Same thing. Disheartening it is to find out how limited I am.
I am returned.
Here again.
Yeah, still lost.
And I am lost again.